Important Name Reactions of Chemistry in Class 12th CBSE and ISC Board
INDEX
- Sandmeyer Reaction
- Gattermann Reaction
- Balz-Schiemann Reaction
- Finkelstein Reaction
- Swartz Reaction
- Wurtz Reaction
- Fitting Reaction
- Wurtz – Fittig Reaction
- Kolbe’s Reaction
- Reimer- Tiemann Reaction
- Rosenmund Reduction
- Gattermann – Koch Reaction
- Stephen Reaction or Stephen Reduction
- Clemmensen Reduction
- Wolff – Kishner Reduction
- Haloform Reaction (Iodoform Reaction)
- Aldol Condensation
- Cannizzaro Reaction
- Friedel – Crafts Reaction
- Grignard Synthesis
- Esterification Reaction or Fischer Esterification
- Williamson Synthesis
- Diazotisation Reaction
- Etard Reaction
- Hell – Volhard Zelinsky Reaction
- Decarboxylation Reaction
- Hofmann Bromide Reaction
- Gabriel Phthalimide Synthesis
- Coupling Reaction
- Carbylamine Reaction
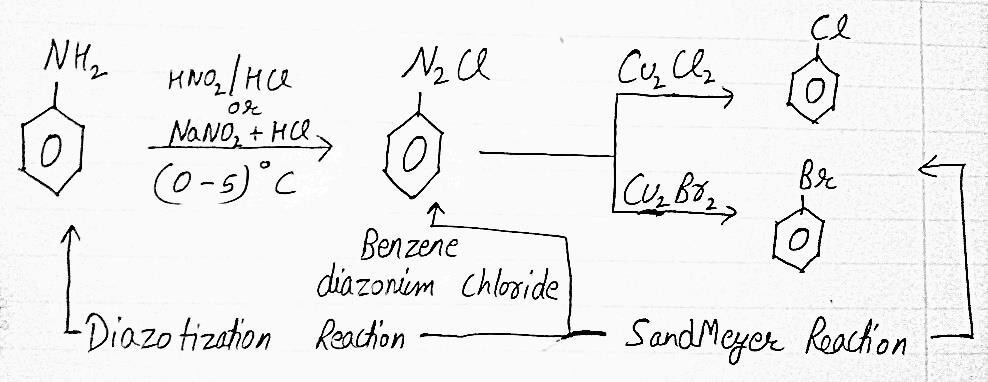
1. SANDMEYER REACTION
The DIAZONIUM (C6H5N2Cl) is prepared by treating ice cold solution of ANILINE (C6H5NH2) in excess of dilute HCl with an aqueous solution of NaNO2 at low temperature (0-5)°C and the reaction is known as DIAZOTIZATION REACTION. BROMO and CHLORO ALKANES can be prepared by treating a freshly prepared DIAZONIUM SALT with CUPPEROUS BROMIDE or CUPPEROUS CHLORIDE and
this reaction is called as SANDMEYER REACTION.
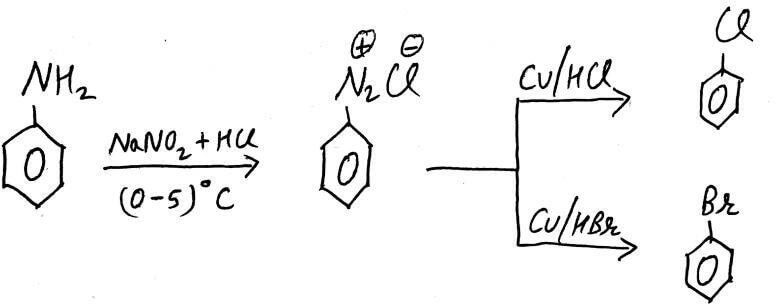
2. GATTERMANN REACTION
This Reaction is same as SANDMEYER REACTION. But the only difference between both of them is that here we use COPPER POWDER (Cu) in the presence of HCl/HBr, and in SANDMEYER REACTION we use Cu2Cl2/Cu2Br2 as catalyst.
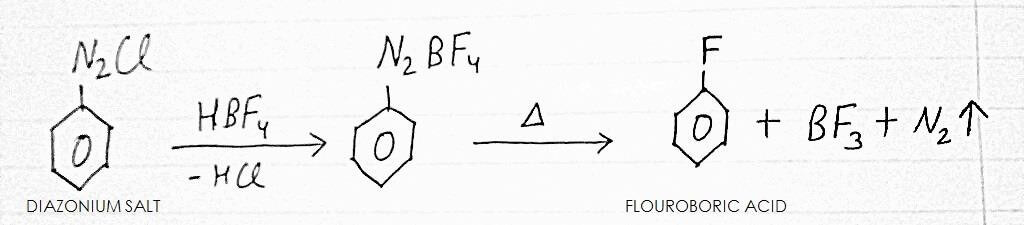
3. BALZ-SCHIEMANN REACTION
It is a method of preparation of FLOUROBENZENE. In this method DIAZONIUM SALT is reacted with FLOUROBORIC ACID, followed by heating the complex compound formed.
4. FINKELSTEIN REACTION
ALKYL IODIDE (R-I) are often prepared by the Reaction of ALKYL CHLORIDE (R- Cl) or ALKYL BROMIDE (R-Br) with SODIUM IODIDE (NaI) in Dry Acetone.
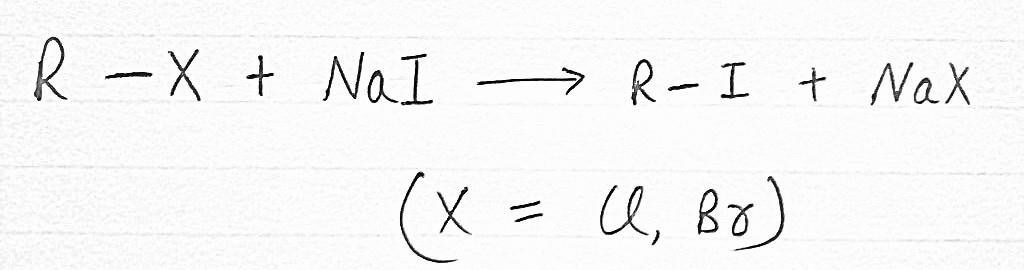
5. SWARTZ REACTION
FLOUROALKANE (ex. – CH3CH2F) are prepared by treating ALKYL CHLORIDE (ex. – CH3CH2Cl) or BROMIDE (ex. – CH3CH2Br) in the presence of metallic FLOURIDES such as AgF, Hg2F2, CoF2 etc.
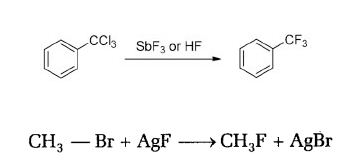
6. WURTZ REACTION
ALKYL HALIDE/HALOALKANE react with metallic SODIUM in the presence of DRY ETHER to form ALKANE containing double the no. of CARBON atom as present in parent ALKYL HALIDE.

7. FITTIG REACTION
Aryl Halides prepared with Sodium (Na) in dry ether to give analogous compounds where two aryl groups joined.

8. WURTZ-FITTIG REACTION
When a mixture of Alkyl Halide and Aryl Halide gets treated with sodium in dry ether, we get an Alkyl Arene.
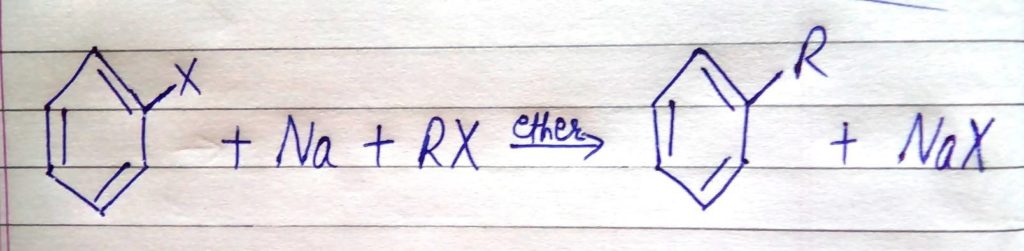
9. KOLBE-SCHMITT REACTION
SODIUM PHENOXIDE (C6H5ONa) when heated with CARBON DIOXIDE (CO2) at
400 Kelvin under a pressure of (4-7)atm followed by acidification gives 2-HYDROXYBENZOIC ACID (SALICYLIC ACID) as the main product. This Reaction is called KOLBE’S REACTION.

10. REIMER TIEMANN REACTION
When PHENOL (C6H5OH) reacts with CHLOROFORM (CHCl3) in the presence of NaOH (SODIUM HYDROXIDE), a CHO group is introduced at ortho position of the Ring. This Reaction is known as REIMER TIEMANN REACTION.

11. Roesnmund Reaction
The Rosenmund Reduction is a hydrogenation process in which an Acyl Chloride is selectively reduced to an Aldehyde. The reaction is catalysed by Palladium on Barium Sulphate.

12. GATTERMAN-KOCH REACTION
When Benzene or its derivative is treated with Carbon Monoxide (CO) and Hydrogen Chloride (HCl) in the presence of Anhydrous AlCl3 or CuCl2, it gives Benzaldehyde or substituted benzaldehyde. This Reaction is called Gatterman Koch Reaction.

13. STEPHEN REACTION
When Ethereal solution of
a Nitrile is reduced with STANNOUS CHLORIDE (SnCl2) in
the presence of HYDROCHLORIC ACID (HCl) at
room temperature, Imine Hydrochloride
(R–CH=NH.HCl) is formed. This up on Hydrolysis with boiling H2O
give Aldehyde and the Reaction is
called STEPHEN REACTION.

14. CLEMMENSEN REDUCTION
Aldehyde and Ketone are reduced to corresponding hydrocarbon when they are reacted with a mixture of Zinc Mercury Alloy and Concentrated HCl. This reaction is called as Clemmensen Reduction.

15. WOLFF – KISHNER REDUCTION
Carbonyl group of Aldehydes and Ketones on treatment with Hydrazine which on heating with Potassium Hydroxide in high boiling solvent (ethylene glycol) reduced to CH2 group.

17. ALDOL CONDENSATION
In this Reaction two Molecules of an ALDEHYDE or a KETONE condense in presence of a Dilute ALKALI (Dilute NaOH, Na2CO3, Ba(OH)2 etc.) to form a
b-hydroxyaldehyde or b-hydroxyketone resp. These b-hydroxyaldehydes or ketones are collectively called ALDOLS.

18. CANNIZZARO REACTION
ALDEHYDES which do not contain a a–hydrogen atom when treated with concentrated ALKALI SOLUTION (NaOH) undergo disproportionation reaction,
i.e. self-Oxidation/Reduction. As a result one molecule is oxidized to
CARBOXYLIC ACID and another molecule is reduced to ALCOHOL.

22. WILLIAMSON SYNTHESIS
It is an important Laboratory method for the preparation of Symmetrical and Unsymmetrical ETHER. In this method an ALKYL HALIDE is allowed to react with SODIUM ALKOXIDE.

24. ETARD REACTION
Chromyl chloride oxidises methyl group to get chromium complex which on hydrolysis provides corresponding benzaldehyde.

25. HELL – VOLHARD ZELINSKY (HVZ) REACTION
Carboxylic acids having an α-hydrogen are halogenated at
the α-position on treatment with chlorine (Cl) or bromine (Br) in the presence
of small amount of red phosphorus to give α-halocarboxylic acid. This reaction
is known as HVZ reaction.

27. HOFMANN BROMIDE REACTION
When a Primary AMIDE is treated with an Aqueous or Alcoholic NaOH (SODIUM HYDROXIDE) or KOH (POTASSIUM HYDROXIDE) solution and BROMINE, it gives a Primary AMINE which has one CARBON atom less than the original AMIDE.

28. GABRIEL PHTHALIMIDE SYNTHESIS
Phthalimide prepared with Ethanolic Potassium Hydroxide produces Potassium salt of Phthalimide when heated with Alkyl Halide followed by Alkaline hydrolysis forms the corresponding primary Amine.

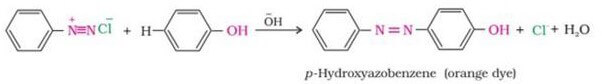
29. COUPLING REACTION
Benzene diazonium chloride gets reacted with phenol in which the phenol molecule at its para position is mixed with the diazonium salt to give p- hydroxyazobenzene.
30. CARBYLAMINE REACTION
Primary amine (both aliphatic and aromatic) when warmed with chloroform and alcoholic KOH, gives isocyanides (carbylamines). This is called carbylamine reaction. Carbylamines has an offensive smell. This reaction is answered only by primary amine and hence to distinguish primary amine from other classes of amines.

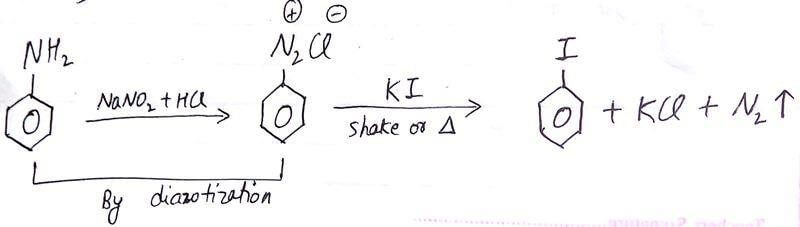
Preparation of Iodobenzene
Replacement of the DIAZONIUM Group by IODINE is done simply by Shaking the
DIAZONIUM SALT with KI.

In Aldol condensation
No. 17
H is missing in Beta carbon with OH
pls check
IF HYDROGEN IS NOT WRITTEN IT IS OBVIOUS THERE. 🙏
sir paper se 2 din phele pass kise ho sakte h agar humko kuch nahi aata to please tell
Continuous reading of past exam papers and related data provides valuable insights into the patterns, types of questions asked, and key topics frequently covered.
In aldol Condensation you can further heat it and form double bond eliminating H2O. Isn’t it the part of the reaction.
quite helpfull , thanks a lot