CPCB Scientist B Exam Pattern
The CPCB Scientist B exam is a computer-based test (CBT) that is conducted by the Central Pollution Control Board (CPCB) to recruit candidates for the post of Scientist B in various disciplines such as Chemistry, Microbiology, and Environmental Science. The exam consists of two parts: Part I and Part II.
Part I of the exam consists of 100 multiple-choice questions (MCQs) that are divided into four sections: General Intelligence and Reasoning, General Awareness, Quantitative Aptitude, and English Language and Comprehension. Each section consists of 25 questions, and the total marks for this part are 100. The duration of Part I is 90 minutes.
Part II of the exam consists of 120 MCQs that are related to the specific discipline for which the candidate has applied. The duration of Part II is 120 minutes, and the total marks for this part are 300.
The total duration of the exam is 210 minutes, and the total marks for the exam are 400. The exam is conducted in English only, and there is a negative marking of one-third of the marks assigned to a question for each incorrect answer.
General Chemistry Mock test
Quiz-summary
0 of 82 questions completed
Questions:
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
- 46
- 47
- 48
- 49
- 50
- 51
- 52
- 53
- 54
- 55
- 56
- 57
- 58
- 59
- 60
- 61
- 62
- 63
- 64
- 65
- 66
- 67
- 68
- 69
- 70
- 71
- 72
- 73
- 74
- 75
- 76
- 77
- 78
- 79
- 80
- 81
- 82
Information
If you can answer all the questions to the Previous Questions chemistry quiz, a million dollars won’t fall down from the skies. However, you’ve got a good chance of making that much money as a chemist!.
|
You must specify a text. |
|
|
You must specify an email address. |
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading...
You must sign in or sign up to start the quiz.
You have to finish following quiz, to start this quiz:
Results
0 of 82 questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 points, (0)
| Average score |
|
| Your score |
|
Categories
- Not categorized 0%
- Bio-Inorganic Chemistry 0%
- inorganic chemistry 0%
-
Here is Your Results!
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
- 46
- 47
- 48
- 49
- 50
- 51
- 52
- 53
- 54
- 55
- 56
- 57
- 58
- 59
- 60
- 61
- 62
- 63
- 64
- 65
- 66
- 67
- 68
- 69
- 70
- 71
- 72
- 73
- 74
- 75
- 76
- 77
- 78
- 79
- 80
- 81
- 82
- Answered
- Review
-
Question 1 of 82
1. Question
1 pointsWhich of the following is not a correct statement about Bitumen?
-
Question 2 of 82
2. Question
2 pointsThe common feature (s) of Rb+, Kr and Br– is /are that they:
a) Have same number of valence electrons.
b) Have same magnitude of effective nuclear charge.
c) Have same magnitude of first Ionization potential.
d) Are iso-electronic species -
Question 3 of 82
3. Question
2 pointsAmong the following the iso-electronic pair is
- NO and CO
- O2–(superoxide) and NO–
- NO+ and CO
- O2–(superoxide) and NO+
-
Question 4 of 82
4. Question
2 points1. The most polar compound amongs the following is
- SF4
- BF3
- XeF4
- SO3
-
Question 5 of 82
5. Question
2 pointsThe lattice energy of LiF calculated from Born Lande-equation is –1000 kJmol–1 . Assume that for both LiF and MgO the modelung constants, interionic distance and Born exponents have the same value.
The lattice energy of MgO in kJmol–1 is
-
Question 6 of 82
6. Question
2 pointsIn the structure of B4O5 (OH)42- -
Question 7 of 82
7. Question
2 pointsB2 H6 and B4 H10 respectively, are example of
-
Question 8 of 82
8. Question
2 pointsThe compound having a S–S single bond is:
- H2S2O3
- H2S2O4
- H2S2O7
- H2S2O8
-
Question 9 of 82
9. Question
2 pointsA chemical compound [X] is used for water softening to remove temporary hardness [X] reacts with Na2CO3 to form caustic soda. When CO2 is bubbled through ‘X’ it becomes cloudy. The chemical formula of ‘X’ is
-
Question 10 of 82
10. Question
2 pointsThe correct order of acidic strength of CrO3 , CrO2 , Cr2O3 and CrO
- CrO2 > CrO3 > Cr2O3 > CrO
- CrO3 < CrO2 < Cr2O3 < CrO
- CrO3 > CrO2 > Cr2O3 > CrO
- CrO2 > Cr2O3 > CrO > CrO3
-
Question 11 of 82
11. Question
2 pointsThe CFSE ( crystal field stabilization energy) in units of D0for [CoF3(H2O)3]
-
Question 12 of 82
12. Question
2 pointsThe correct order of d-orbital splitting in trigonal prismatic geometry is
- dz2>dx2–y2, dxy>dxz, dyz
- dxz, dyz > dx2 – y2 , dxy > dz2
- dxz, dyz > dz2 > dx2 – y2 , dxy
- dz2 > dxy, dzy > dx2 – y2.dxy
-
Question 13 of 82
13. Question
2 pointsWhich of the following complex ions absorbs the light of minimum wavelength?
A .[Co(H2O)6]3+
B .[CoF6]3−
C .[Co(CN)6]3−
D .[Co(NH3)6]3+
-
Question 14 of 82
14. Question
2 pointsWhich statement is correct about a 1H NMR spectrum of 99% deuterium-enriched CDCl3?
-
Question 15 of 82
15. Question
2 pointsWhich molecule is non-polar?
-
Question 16 of 82
16. Question
2 pointsWhich statement is incorrect?
-
Question 17 of 82
17. Question
2 pointsWhich of the following is a saline hydride?
-
Question 18 of 82
18. Question
2 pointsWhich of the following has a polymeric structure in the solid state?
-
Question 19 of 82
19. Question
2 pointsWhat is the coordination number of Al in the solid state structure of AlH3?
-
Question 20 of 82
20. Question
2 pointsWhich statement is incorrect about the reaction of N2 and H2 to give NH3?
-
Question 21 of 82
21. Question
2 pointsWhich statement is incorrect?
-
Question 22 of 82
22. Question
2 pointsIn which reaction is H2 liberated?
-
Question 23 of 82
23. Question
2 pointsWhich pair of solvents, X and Y, is expected to show classical intermolecular hydrogen bonding between X and Y?
-
Question 24 of 82
24. Question
2 pointsWhich of the following reactions does not produce H2?
-
Question 25 of 82
25. Question
2 pointsWhich of the following molecules possesses a dipole moment?
-
Question 26 of 82
26. Question
2 pointsWhich metal does not liberate H2 from dilute aqueous hydrochloric acid at 298 K?
-
Question 27 of 82
27. Question
4 pointsWhich of the following transitions of electrons in the hydrogen, atom will emit maximum energy ?
-
Question 28 of 82
28. Question
1 pointsFor a reaction of the type A + B → Products, the unit of the rate constant is mol L–1 s–1.The overall order of the reaction is
-
Question 29 of 82
29. Question
1 pointsThe thermodynamic criterion for spontaneity of a process in a system under constantvolume and temperature and in the absence of any work other than expansion work (if any)is
-
Question 30 of 82
30. Question
1 pointsThe number of vibrational mode(s) of a carbon dioxide molecule that can be detected using infrared spectroscopy is
-
Question 31 of 82
31. Question
1 pointsFor three non-coplanar vectors a, b and c, the expression a ∙ (b × c) can be written as
-
Question 32 of 82
32. Question
1 pointsCorrect trend in the bond order is
(A) O2+ >O22- >O2–
(B) O2– >O2+ >O22-
(C) O22- >O2– >O2+
(D) O2+ >O2– >O22-
-
Question 33 of 82
33. Question
1 pointsThe correct option for the metal ion present in the active site of myoglobin, hemocyanin and vitamin B12, respectively, is
-
Question 34 of 82
34. Question
1 pointsThe correct order of wavelength (λmax) of the halide to metal charge-transfer band of [Co(NH3)5Cl]2+ (I), [Co(NH3)5Br]2+ (II) and [Co(NH3)5I]2+ (III), is
-
Question 35 of 82
35. Question
1 pointsThe correct option for the major products of the following reaction is
(image) Q8
-
Question 36 of 82
36. Question
1 pointsThe major product formed in the following reaction is
Q9 IMAGE
Q9A
Q9B
Q9C
Q9D
-
Question 37 of 82
37. Question
1 pointsThe complementary strand for the following single strand of DNA is
5’<-A-T-C-G-T->3’
-
Question 38 of 82
38. Question
1 pointsThe function f(x) x e-x2 has a minimum at
A) x = √2
B) x = −√2
C) x = 1/√2
D) x = −1/√2
-
Question 39 of 82
39. Question
1 pointsThe correct option for the number of bending modes of vibration in each of H2O, CS2 and SO2 molecules, respectively, is
-
Question 40 of 82
40. Question
1 pointsThe total number of degrees of freedom of an HBr molecule that is constrained to translate along a straight line but does not have any constraints for its rotation and vibration is
-
Question 41 of 82
41. Question
1 pointsAccording to the kinetic theory of gases, the ratio of the root mean square velocity of molecular oxygen and molecular hydrogen at 300 K is
-
Question 42 of 82
42. Question
1 pointsThe half-life of the chemical reaction, A → Product, for initial reactant concentrations of 0.1 and 0.4 mol L–1 are 200 and 50 s, respectively. The order of the reaction is
-
Question 43 of 82
43. Question
1 pointsThe ratio of the nearest neighbor atomic distances in body-centered cubic (bcc) and facecentered cubic (fcc) crystals with the same unit cell edge length is
-
Question 44 of 82
44. Question
1 pointsThe correct trend in the rate of substitution of Cl- by pyridine in the following complexes is
IMAGE Q17
-
Question 45 of 82
45. Question
1 pointsIn qualitative inorganic analysis of metal ions, the ion which precipitates as sulfide in the presence of H2S in warm dilute HCl is
1- Cr3+
2- Al3+
3- Co2+
4- Bi3+
-
Question 46 of 82
46. Question
1 pointsThe correct statement regarding the observed magnetic properties of NO, O2, B2, and C2 in their ground state is
NO, B2, and C2 are paramagnetic ——–1
B2, O2 and NO are paramagnetic———2
O2, C2 and NO are paramagnetic———3
O2, B2 and C2 are paramagnetic———-4
-
Question 47 of 82
47. Question
1 pointsThe observed magnetic moments of octahedral Mn3+, Fe3+ and Co3+ complexes are 4.95, 6.06 and 0.00 BM, respectively. The correct option for the electronic configuration of Mn3+, Fe3+ and Co3+ metal ions in these complexes, respectively, is
IMAGE
-
Question 48 of 82
48. Question
1 pointsAmong the following compounds, the one having the lowest boiling point is
A) SnCl4
B) GeCl4
C) SiCl4
D) CCl4
-
Question 49 of 82
49. Question
1 pointsThe correct option having one complex from each of the following pairs which is more reactive towards the oxidative addition reaction by hydrogen molecule is
Pair 1: IrCl(PMe3)3 (I) and IrCl(CO)(PMe3)2 (II)
Pair 2: IrCl(CO)(PPh3)2 (III) and IrCl3(PPh3) (IV) -
Question 50 of 82
50. Question
1 pointsAmong the following, the correct statement is
A. The density follows the order, Cs > Rb > Li > Na.
B. The solubility in water follows the order, Cs2CO3 > K2CO3 > Na2CO3 > Li2CO3.
C. The first ionization potential follows the order, Li > K > Na > Cs.
D. The melting point follows the order, MgCl2 > BeCl2 > CaCl2 > SrCl2.
-
Question 51 of 82
51. Question
1 pointsThe major product of the following reaction is
IMAGE 24 WITH 24A 24B 24C 24D
-
Question 52 of 82
52. Question
1 pointsIn 1H NMR spectrum of the given molecule, the correct order of chemical shifts of the labelled protons (HX, HY, HZ) is
IMAGE Q25
A) HZ > HX > HY
B) HZ > HY > HX
C) HX > HY > HZ
D) HY > HX > HZ
-
Question 53 of 82
53. Question
1 pointsIn the following reaction of (D)-Glucose, a product P is formed.

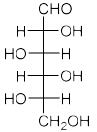
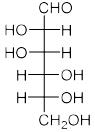
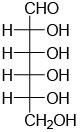
Among the following compounds, the one which will give the same product (P) under identical reaction conditions isA

B

C

D

-
Question 54 of 82
54. Question
1 pointsThe major product of the following reaction is IMAGE Q27
IMAGE 27A
IMAGE 27B
IMAGE 27C
IMAGE 27D
-
Question 55 of 82
55. Question
1 pointsThe correct option for the product(s) of the following reaction is
IMAGE Q28
IMAGE 28B
IMAGE 28C
IMAGE 28D
-
Question 56 of 82
56. Question
1 pointsThe increasing order of acidity of the given molecules in aqueous media is
IMAGE Q29
-
Question 57 of 82
57. Question
1 pointsThe compound formed upon subjecting an aliphatic amine to Lassaigne’s test is
-
Question 58 of 82
58. Question
1 pointsThe eigenvalue(s) of the matrix is/are
IMAGE Q31
-
Question 59 of 82
59. Question
1 pointsThe unit of the constant ‘a’ in van der Waals equation of state of a real gas can be expressed as
A. m6 Pa mol-2
B. m6 J mol-2
C. m3 Pa mol-2
D. m3 J mol-2
-
Question 60 of 82
60. Question
1 pointsAmong the following, microwave active molecule(s) is/are
-
Question 61 of 82
61. Question
1 pointsThe true statement(s) regarding the brown ring test carried out in the laboratory for the detection of NO3– is/are
A) Brown ring is due to the formation of the iron nitrosyl complex.
B) Concentrated nitric acid is used for the test.
C) The complex formed in the reaction is [Fe(CN)5NO]2-.
D) The brown colored complex is paramagnetic in nature.
-
Question 62 of 82
62. Question
1 pointsThe true statement(s) regarding the carbonic anhydrase enzyme is/are
-
Question 63 of 82
63. Question
1 pointsThe correct statement(s) about NO2, NO2+ and CO2 is /are
A. Both NO2 and CO2 are paramagnetic.
B. NO2 is paramagnetic and NO2+ is diamagnetic
C. Both CO2 and NO2+ have linear geometry.
D. CO2 and NO2+ are isoelectronic.
-
Question 64 of 82
64. Question
1 pointsThe compound(s) formed as intermediate(s) in the following reaction sequence is/are
IMAGE Q37 PLUS ALL OPTIONS IMG
-
Question 65 of 82
65. Question
1 pointsThe correct statement(s) among the following is/are
-
Question 66 of 82
66. Question
1 pointsThe diastereomeric pair(s) among the following option(s) is/are
OPTIONS IMAGE 39ABCD
-
Question 67 of 82
67. Question
1 pointsThe reaction(s) that result(s) in the formation of aromatic species is/are
IMAGE OPTIONS 40ABCD
-
Question 68 of 82
68. Question
2 pointsWhich statement is incorrect?
-
Question 69 of 82
69. Question
2 pointsWhich of the following is a saline hydride?
-
Question 70 of 82
70. Question
2 pointsWhich of the following has a polymeric structure in the solid state?
-
Question 71 of 82
71. Question
2 pointsWhich statement is incorrect about the reaction of N2 and H2 to give NH3?
-
Question 72 of 82
72. Question
2 pointsWhich statement is incorrect?
-
Question 73 of 82
73. Question
2 pointsWhich pair of solvents, X and Y, is expected to show classical intermolecular hydrogen bonding between X and Y?
-
Question 74 of 82
74. Question
2 pointsWhich of the following reactions does not produce H2?
-
Question 75 of 82
75. Question
2 pointsWhich of the following molecules possesses a dipole moment?
-
Question 76 of 82
76. Question
2 pointsWhich metal does not liberate H2 from dilute aqueous hydrochloric acid at 298 K?
-
Question 77 of 82
77. Question
2 pointsWhich molecule is non-polar?
-
Question 78 of 82
78. Question
2 pointsWhen a reduced cytochrome transfers an electron from its Fe(II) to the bound O2
-
Question 79 of 82
79. Question
2 pointsThe lattice energy of LiF calculated from Born Lande-equation is –1000 kJmol–1 . Assume that for both LiF and MgO the modelung constants, interionic distance and Born exponents have the same value.
The lattice energy of MgO in kJmol–1 is
-
Question 80 of 82
80. Question
2 pointsIn the structure of B4O5 (OH)42- -
Question 81 of 82
81. Question
2 pointsThe ligand system present in vitamin B12 is,
-
Question 82 of 82
82. Question
2 pointsWhich molecule is non-polar?
Scoreboard
Leaderboard: General Chemistry Mock test
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
Most Asked MCQs
1. Which of the following air pollutants is responsible for the formation of acid rain?
a. Carbon monoxide
b. Nitrogen dioxide
c. Sulfur dioxide
d. Ozone
2. What is the primary source of indoor air pollution in homes?
a. Outdoor air pollution
b. Tobacco smoke
c. Cooking fumes
d. Pet dander
3. The Bhopal Gas Tragedy of 1984 was caused by a leak from which of the following industries?
a. Textile
b. Chemical
c. Petroleum
d. Steel
4. Which of the following pollutants is primarily responsible for the depletion of the ozone layer?
a. Sulfur dioxide
b. Carbon dioxide
c. Chlorofluorocarbons (CFCs)
d. Nitrogen oxides
5. Which of the following is a commonly used method for treating industrial wastewater?
a. Reverse osmosis
b. Activated sludge process
c. Chemical precipitation
d. Biological oxidation
6. The National Green Tribunal (NGT) was established in which year?
a. 2005
b. 2010
c. 2015
d. 2020
7. Which of the following is an example of non-biodegradable waste?
a. Food waste
b. Paper waste
c. Plastic waste
d. Garden waste
8. The Indian government’s Swachh Bharat Abhiyan campaign aims to achieve which of the following objectives?
a. Encouraging sustainable tourism
b. Improving access to healthcare
c. Promoting cleanliness and hygiene
d. Reducing poverty and inequality
9. The Air Quality Index (AQI) measures the concentration of which of the following pollutants in the air?
a. Carbon dioxide
b. Nitrogen dioxide
c. Ozone
d. All of the above
10. The Solid Waste Management Rules were first introduced in which year?
a. 2000
b. 2005
c. 2010
d. 2016
11. Which of the following is a greenhouse gas that is produced by agricultural activities such as livestock farming?
a. Methane
b. Carbon monoxide
c. Sulfur dioxide
d. Nitrogen oxides
12. Which of the following is a renewable source of energy?
a. Coal
b. Natural gas
c. Wind
d. Petroleum
13. The World Health Organization (WHO) has established guidelines for the safe level of which of the following air pollutants?
a. Carbon monoxide
b. Sulfur dioxide
c. Nitrogen dioxide
d. Particulate matter
14. The National River Conservation Plan was launched in which year?
a. 1985
b. 1995
c. 2005
d. 2015
15. The Ozone Depleting Substances (Regulation and Control) Rules were first introduced in which year?
a. 1995
b. 2000
c. 2005
d. 2010
16. Which of the following is a commonly used method for controlling water pollution?
a. Activated carbon filtration
b. Chlorination
c. Sedimentation
d. Biological treatment
17. Which of the following pollutants is responsible for the phenomenon known as “smog”?
a. Sulfur dioxide
b. Carbon monoxide
c. Nitrogen oxides
d. Ozone
18. The National Clean Energy Fund was established in which year?
a. 2009
b. 2012
c. 2015
d. 2018
19. The Wildlife Protection Act was first introduced in which year?
a. 1965
b. 1972
c. 1985
d. 1995
20. Which of the following is a commonly used method for controlling noise pollution?
a. Constructing sound barriers
b. Planting trees
c. Installing mufflers
d. All of the above
21. The Forest Conservation Act was enacted in which year?
a. 1980
b. 1990
c. 2000
d. 2010
22. The Integrated Coastal Zone Management (ICZM) plan was introduced in which year?
a. 1995
b. 2000
c. 2005
d. 2010
23. Which of the following pollutants is responsible for the formation of smog in Delhi during winter months?
a. Carbon dioxide
b. Sulfur dioxide
c. Nitrogen dioxide
d. Particulate matter
24. The Paris Agreement on climate change was adopted in which year?
a. 2013
b. 2014
c. 2015
d. 2016
25. The National Air Quality Index (NAQI) was launched in which year?
a. 2012
b. 2014
c. 2016
d. 2018
26. The Indian government’s National Action Plan on Climate Change (NAPCC) was launched in which year?
a. 2007
b. 2008
c. 2009
d. 2010
27. Which of the following is a commonly used method for controlling air pollution from industries?
a. Scrubbers
b. Activated carbon filtration
c. Chlorination
d. Sedimentation
28. The Wildlife Protection Act provides legal protection to which of the following species?
a. Endangered plants
b. Endangered animals
c. Both A and B
d. None of the above
29. Which of the following is a commonly used method for controlling soil erosion?
a. Contour ploughing
b. Use of chemical fertilizers
c. Deforestation
d. Overgrazing
30. The Indian government’s National River Conservation Directorate is under which ministry?
a. Ministry of Environment, Forest and Climate Change
b. Ministry of Water Resources, River Development and Ganga Rejuvenation
c. Ministry of Science and Technology
d. Ministry of Power
31. The Global Environment Facility (GEF) was established in which year?
a. 1985
b. 1990
c. 1995
d. 2000
32. The United Nations Framework Convention on Climate Change (UNFCCC) was signed in which year?
a. 1987
b. 1992
c. 1997
d. 2002
33. The National Green Tribunal (NGT) was established in which year?
a. 2010
b. 2011
c. 2012
d. 2013
34. Which of the following is a commonly used method for controlling indoor air pollution?
a. Use of air purifiers
b. Regular cleaning
c. Proper ventilation
d. All of the above
35. The National Biodiversity Act was enacted in which year?
a. 1995
b. 2000
c. 2005
d. 2010
36. Which of the following is a commonly used method for solid waste management?
a. Landfills
b. Incineration
c. Recycling
d. All of the above
37. The Wetland (Conservation and Management) Rules were first introduced in which year?
a. 1995
b. 2000
c. 2010
d. 2017
38. The National Adaptation Fund for Climate Change (NAFCC) was established in which year?
a. 2007
b. 2010
c. 2015
d. 2020
39. The National Clean Energy Fund was renamed as the National Clean Energy and Environment Fund in which year?
a. 2012
b. 2014
c. 2016
d. 2018
40. The Sustainable Development Goals (SDGs) were adopted by the United Nations in which year?
a. 2012
b. 2014
c. 2015
d. 2016
41. Which of the following is a greenhouse gas that is emitted by rice paddies?
a. Carbon dioxide
b. Methane
c. Nitrous oxide
d. All of the above
42. Which of the following is a commonly used method for controlling water pollution from industries?
a. Coagulation-flocculation
b. Reverse osmosis
c. Ion exchange
d. All of the above
43. The National Wildlife Action Plan was launched in which year?
a. 1995
b. 2000
c. 2005
d. 2010
44. The Swachh Bharat Abhiyan was launched in which year?
a. 2012
b. 2014
c. 2016
d. 2018
45. The National Greenhouse Gas Inventory was first prepared in which year?
a. 1995
b. 2000
c. 2005
d. 2010
46. The National River Conservation Plan was launched in which year?
a. 1985
b. 1990
c. 1995
d. 2000
47. Which of the following is a commonly used method for controlling noise pollution?
a. Use of earplugs
b. Sound barriers
c. Noise insulation
d. All of the above
48. The Forest Rights Act was enacted in which year?
a. 2006
b. 2008
c. 2010
d. 2012
49. The National Wetland Atlas was released in which year?
a. 2000
b. 2005
c. 2010
d. 2015
50. The National Action Plan for Conservation of the Critically Endangered Species was launched in which year?
a. 2017
b. 2018
c. 2019
d. 2020
Answer key
1. c
2. a
3. d
4. b
5. c
6. a
7. d
8. c
9. b
10. d
11. b
12. d
13. a
14. b
15. c
16. b
17. c
18. d
19. a
20. b
21. d
22. c
23. b
24. c
25. a
26. b
27. d
28. c
29. a
30. d
31. b
32. b
33. c
34. d
35. d
36. d
37. b
38. b
39. b
40. c
41. b
42. d
43. a
44. b
45. a
46. a
47. d
48. b
49. d
50. b


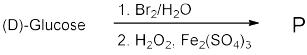
Answers are wrong